Tracer-QC receives regulatory approval in Europe
15 April 2024

After extensive testing by Haukeland University Hospital, the automated PET-QC system has proven equivalency with traditional QC methods for 18F-FDG
Following a validation submission by Haukeland University Hospital to the Norwegian Medical Products Agency (DMPA), twelve analytical methods running on the Tracer-QC platform have been approved for the Quality Control of 18F-FDG intended for clinical use. This follows the recent announcement that Tracer-QC is being routinely used in the production of 18F-FDG to standards defined by US Pharmacopeia at the International Medical Center.
Tracer-QC is the first successful automated solution for PET radiopharmaceutical QC, being a single-instrument solution that performs multiple tests in just 5 clicks of a mouse. DMPA is widely regarded as a highly reputable regulatory authority in Europe. Its endorsement of Tracer-QC methods for supporting the pharmacopeial quality of 18F-FDG sets an example for other countries following the European Pharmacopeia standards.
ICH compliance
DMPA complies with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), as do other authorities in the US and Europe, meaning Tracer-QC analytical methods have met strict international criteria.
Since November 2021, Haukeland has been working with Tracer-QC to prove its validity in 18F-FDG production. Head of Quality Control Ole Heine Kvernenes said, “DMPA looked extensively at all the different tests that Tracer-QC carried out. It is always a challenge to be the first one to adopt a new system, but Tracer-QC’s potential to do everything all at once appealed to us and we believed it could do what it was supposed to. Besides FDG, we intend to validate and use Tracer-QC for other tracers in our portfolio.”
“Automation has got to come.”
Ole said, “More and more hospitals want to use FDG, so instead of having a huge QC laboratory filled with lots of pieces of different equipment, it’s much easier to have a single apparatus that can do it all for you. Automation has got to come.
“Tracer-QC is taking existing analytical methods and applying smart thinking and sensible design. Once you understand that the endotoxin test it performs is just a regular endotoxin test, it doesn’t seem so strange. It’s using many already approved methods. It’s mainly the concept of coming in one single package that’s new.”
Proven equivalency with traditional QC
Trace-Ability President and Founder Arkadij Elizarov said, “We have been developing Tracer-QC in partnership with LabLogic for a while now, and this approval by the Norwegian authorities is our biggest milestone to date. Equivalency of the twelve Tracer-QC analytical methods used at Haukeland to those used in traditional QC testing has been demonstrated.
“For a long time, Tracer-QC was a curiosity in nuclear medicine. People were intrigued by the concept but sceptical, and the question of regulatory compliance was always the first question that came up with customers. But between Haukeland and IMC, we’ve demonstrated that Tracer-QC is not just a demonstration anymore, it’s a practical application with real-world use.”
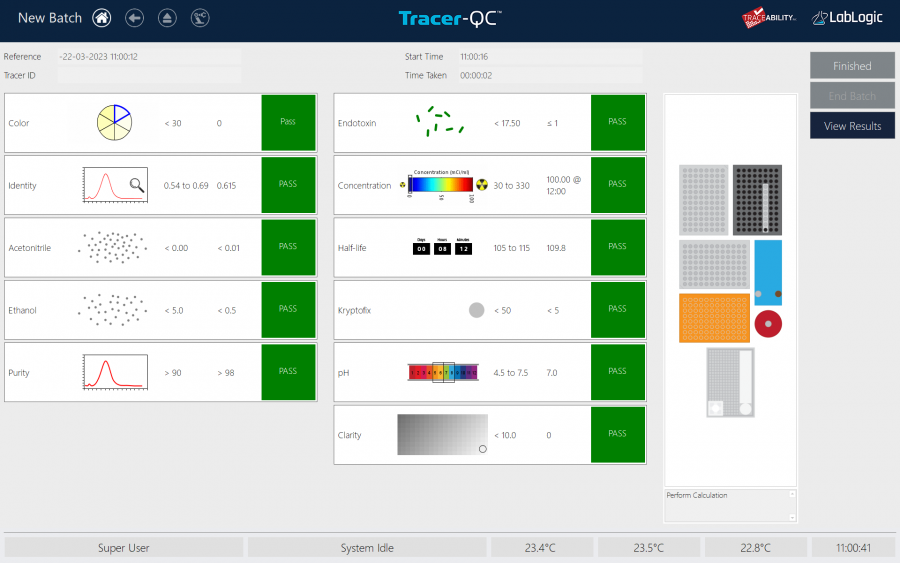
Tracer-QC's tests.
The first proven solution brought to market
“I have always had the conviction that automation was the way forward, it just simply wasn’t possible before. The industry has been trying to achieve this for a number of years, and many big players have invested significant resources into automating QC only to ultimately fail, and so I am immensely proud of what Trace-Ability and LabLogic have achieved and that it was us that have brought the first proven solution to market.”
What it means for other potential customers
“Because Tracer-QC methods comply with ICH and European Pharmacopiea, DMPA’s approval should give customers in Europe and further afield the confidence that they too can do it. We’ve faced scepticism before, but we continue to address it.
“As many know, we are working with the FDA and early adopters towards approval in the US. For those customers keen to benefit from Tracer-QC in the US, this news that Tracer-QC satisfies the same ICH requirements that the FDA adheres to should also give them a confidence that it is positioned for approval in the US in due time.”
Improving access to PET radiotracers
LabLogic Sales Director Elvir Zahirovic said, “This significant milestone is thanks to the exceptional expertise and talent of Ole and his team at Bergen, our partners at Trace-Ability, and my colleagues at LabLogic. Automated QC testing of PET radiotracers represents a pivotal step in the optimisation and standardisation of processes by addressing the bottlenecks that traditionally required highly skilled staff. This achievement not only streamlines operations but also holds the promise of improving access to PET tracers. We believe that our Tracer-QC automated QC platform, rigorously tested and accepted by a European medicine agency, will pave the way for Tracer-QC’s broader acceptance within the industry.”
Find out more
You can learn more about Tracer-QC and its automation of the PET-QC process by clicking the button below to speak to a product specialist.